Learn More About Our Psychedelic Studies
Learn More About Our Psychedelic Studies
National Survey Investigating Hallucinogenic Trends (NSIHT)
Summary
NSIHT focuses on individuals actively using psychedelics to inform overall safety, utility, and effectiveness using real-world evidence. This cross-sectional surveillance pilot study intends, over time, to be the most comprehensive study of psychedelic use in the United States. It was informed through focus groups and underwent external expert reviews.
Launched in April of 2024, the pilot survey had 2,300 respondents since April of 2024, capturing the following data:
- Patterns of use
- Reasons for use
- Therapeutic outcomes
- Measures of mental health symptoms
- Adverse events
- Physical/sexual assault
Development of pilot funded through US Substance Abuse and Mental Health Administration (SAMHSA) contract #A23-0105-00_9810
To learn more or for collaboration opportunities & additional survey capabilities, please contact us
Sentinel Poison Center
Summary
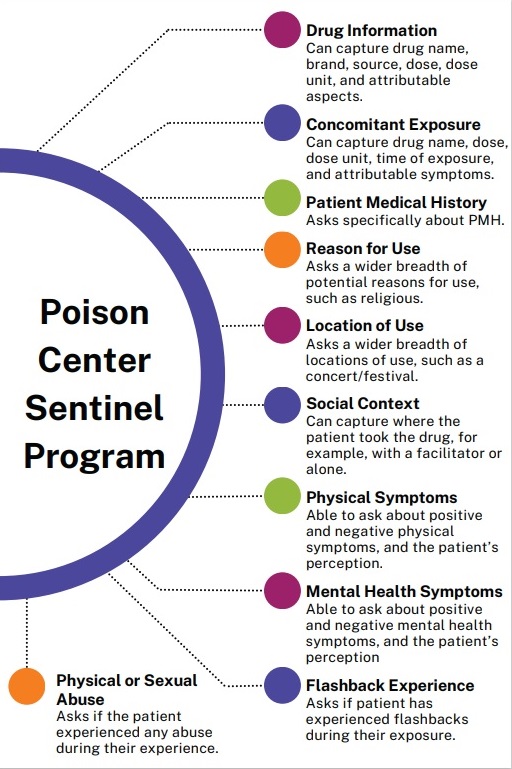
This is a surveillance system of psychedelic drug exposures reported to US Poison Centers. The primary purpose of this study is to quantify and monitor the number of adverse events and medical outcomes associated with psychedelic drug use reported to US poison centers.
This augmented approach to poison center data collection will capture contextual and clinical variables currently not well captured by poison centers.
The Sentinel poison center program is currently collecting data on psychedelic safety in 4 states (Colorado, Hawaii, Nevada, and Montana).
To learn more or for collaboration opportunities, please contact us
Longitudinal Psychedelic Treatment Study - Healing Centers
As new psychedelic drugs emerge, understanding their real-world safety and effectiveness is crucial. Treatments like psilocybin and ketamine are being explored for mental health conditions, with FDA-approved esketamine already available for treatment-resistant depression. While promising, these treatments come with potential risks and benefits that need thorough investigation.
The Healing Centers study is an observational, longitudinal project that will collect patient data over approximately one year. It aims to gather:
- Demographic and medical history to describe participants.
- Pre- and post-treatment data to analyze changes in mental health symptoms and overall wellbeing.
- Quantitative and qualitative experiences to identify any negative outcomes or adverse events.
Key measures will include patient perceptions of health, wellbeing, social support, psychological insight, and serious adverse events like hospitalization.
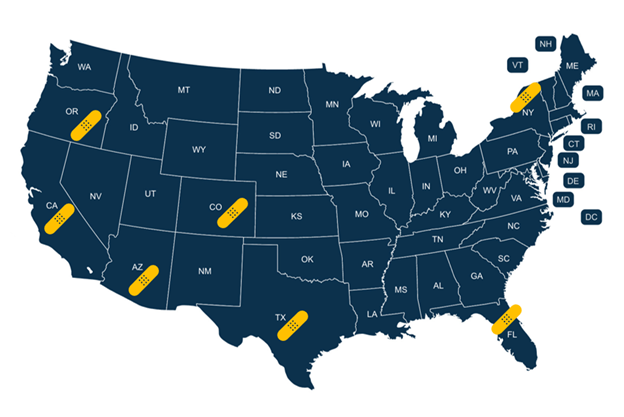
Data collection is active in 15 clinics in 7 states.
Current Site Enrollment Locations:
For more information about our Longitudinal Psychedelic Treatment Study - Healing Centers program or to enroll, please contact us